Product Symbols
& Their Meanings
Wherever possible and appropriate, we use internationally harmonized symbols to communicate important product instructions and precautions. Our labeling is designed to meet the following standards: 21 CFR 820.120, 21 CFR 801, ISO 13485 section 3.6, EN ISO 15223-1, EN ISO 20417, applicable sections of the Canadian Medical Device Regulation and other international standards/regulations. Below is a complete listing of the symbols used.
Symbols Glossary
| Symbol | Symbol Ref. No. | Title of Symbol | |
|---|---|---|---|
| 5.1.1 | Manufacturer | Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC. |
|
| 5.1.2 | Authorized representative in the European Community |
Indicates the authorized representative in the European Community. | |
| 5.1.3 | Date of manufacture | Indicates the date when the medical device was manufactured. | |
| 5.1.4 | Use by date | Indicates the date after which the medical device is not to be used. | |
| 5.1.5 | Batch code | Indicates the manufacturer’s batch code so that the batch or lot can be identified. | |
| 5.1.6 | Catalog number | Indicates the manufacturer’s catalog number so that the medical device can be identified. | |
| 5.1.7 | Serial number | Indicates the manufacturer’s serial number so that a specific medical device can be identified. | |
| 5.1.8 | Importer | Indicates the entity importing the medical device into the locale. | |
| 5.1.9 | Distributor | Indicates the entity distributing the medical device into the locale. | |
| 5.2.3 | Sterilized using ethylene oxide | Indicates a medical device that has been sterilized using ethylene oxide. | |
| 5.2.4 | Sterilized using irradiation | Indicates a medical device that has been sterilized using irradiation. | |
| 5.2.6 | Do not resterilize | Indicates a medical device that is not to be resterilized. | |
| 5.2.7 | Nonsterile | Indicates a medical device that has not been subjected to a sterilization process. | |
| 5.2.8 | Do not use if package is damaged. | Indicates a medical device that should not be used if the package has been damaged or opened. | |
| 5.3.2 | Keep away from sunlight | Indicates a medical device that needs protection from light sources. | |
| 5.3.4 | Keep dry | Indicates a medical device that needs to be protected from moisture. | |
| 5.3.7 | Temperature limit | Indicates the upper and lower limits of temperature to which the medical device can be safely exposed. The temperature is indicated adjacent to the horizontal lines. | |
| 5.3.6 | Upper limit of temperature | Indicates the upper limit of temperature to which the medical device can be safely exposed. The temperature is indicated adjacent to the upper horizontal line. | |
| 5.3.5 | Lower limit of temperature | Indicates the lower limit of temperature to which the medical device can be safely exposed. The temperature is indicated adjacent to the lower horizontal line. | |
| 5.4.1 | Biological risk | Indicates that there are potential biological risks associated with the medical device. | |
| 5.4.2 | Do not reuse | Indicates a medical device that is intended for one use or for use on a single patient during a single procedure. | |
| 5.4.3 | Consult instructions for use | Indicates the need for the user to consult the instructions for use. | |
| 5.4.4 | Caution | Indicates that the instructions for use contain important cautionary information such as warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself. | |
| 5.4.5 | Contains or presence of natural rubber latex | Indicates the presence of natural rubber or dry natural rubber latex as a material of construction within the medical device or the packaging of a medical device, which may cause allergic reactions. | |
| 5.4.7 | Contains a medicinal substance | Indicates a medical device that contains or incorporates a medicinal substance. | |
| 5.4.10 | Contains hazardous substances | Indicates a medical device that contains substances that can be carcinogenic, mutagenic or reprotoxic (CMR), or substances with endocrine-disrupting properties. | |
| 5.4.12 | Single patient multiple use | Indicates a medical device that may be used multiple times (multiple procedures) on a single patient. | |
| 5.6.3 | Non-pyrogenic | Indicates a medical device that is non-pyrogenic. | |
| 5.7.1 | Patient number | Indicates a unique number associated with an individual patient. | |
| 5.7.2 | Patient name | Indicates the name of the patient. | |
| 5.7.3 | Patient identification | Indicates the identification data of the patient. | |
| 5.7.4 | Patient information website | Indicates a website where a patient can obtain additional information on the medical product. | |
| 5.7.5 | Health care center or doctor | To indicate the address of the health care center or doctor where medical information about the patient may be found. | |
| 5.7.6 | Date | To identify the date that information was entered or a medical procedure took place. | |
| 5.7.7 | Medical device | Indicates the item is a medical device. | |
| 5.7.8 | Translation | To identify that the original medical device information has undergone a translation that supplements or replaces the original information. | |
| 5.7.9 | Repackaging | To identify that a modification to the original medical device packaging configuration has occurred. | |
| 5.7.10 | Unique Device Identifier | Indicates a carrier that contains Unique Device Identifier information. | |
| 5.2.11 (ISO 15223-1:2021) | Sterile barrier system | Indicates a single sterile barrier system. | |
| 5.2.12 (ISO 15223-1:2021) | Sterile barrier system | Indicates a double sterile barrier system. | |
| 5.2.13 (ISO 15223-1:2021) | Sterile barrier system | Indicates a single sterile barrier system with protective packaging inside. | |
| 5.2.14 (ISO 15223-1:2021) | Sterile barrier system | Indicates a single sterile barrier system inside protective packaging. | |
 |
ASTM F2503 § 7.4.9 | MR unsafe | Indicates that the device is not MR safe and should remain outside of the 5G field. |
 |
IEC 60417 Symbol 5335 | Applied part | Indicates a type CF Applied part. |
 |
IEC 60529 | Ingress protection code 22 | Vertically dripping water will not cause a hazard. |
 |
ISO 7010 Symbol M002 | ISO 7010-M002 | Refer to instruction manual. |
| Symbol | Standard/ Symbol Reference No. | Title of Symbol | Description of Symbol | |
|---|---|---|---|---|
| RxOnly | 21 CFR 801.109 | The symbol statement for prescription device | Indicates that the product is a medical device as defined in 21 CFR 820.3(l) and federal law (USA) restricts this device to sale by or on the order of a physician (21 CFR 801.109). | |
| European Medical Devices Directive 93/42/EEC June 14, 1993 (as amended by Directive 2007/47/EC), as described in Article 17 of the Directive | Conformité Européene or European conformity | Indicates manufacturer declaration that the product complies with the essential requirements of the relevant European health, safety and environmental protection legislation. | ||
 |
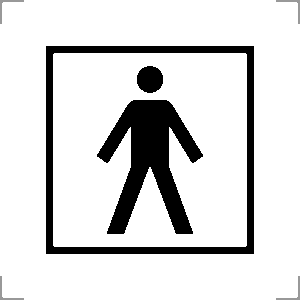
IEC 60417, Graphical symbols for use on equipment Symbol Reference No: 5840 | Type B applied part | Indicates a type B applied part complying with relevant section of the technical standard IEC 60601-1 for safety of medical electrical equipment. | |
 |
IEC 60417, Graphical symbols for use on equipment Symbol Reference No: 5333 | Type BF applied part | Indicates a type BF applied part complying with relevant section of the technical standard IEC 60601-1 for safety of medical electrical equipment. | |
 |
ASTM F2503-13, Standard Practice for Marking Medical Devices and Other Items for Safety In the Magnetic Resonance Environment. As described in Section 7.4.6 of the Standard | MR conditional | Medical device that has been demonstrated to pose no known hazards in a specified MR environment with specified conditions of use. Field conditions that define the MR environment include static magnetic field strength; spatial gradient; and time rate of change of the magnetic field (dB/dt), RF fields, and specific absorption rate (SAR). These conditions are identified on all appropriate product labeling. |
|
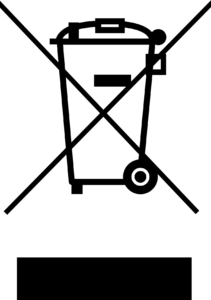 OR OR |
BS EN 50419, Marking of Electrical and Electronic Equipment in accordance with Article 11(2) of the European Community Directive 2002/96/EC (WEEE) | Crossed-out wheelie bin | Identifies product that is subject to the European Union’s Waste Electrical and Electronic Equipment (WEEE) 2012/19/EU Directive for recycling of electronic equipment. The black bar underneath the bin indicates goods that were placed on the market after August 13, 2005. |
|
 |
BS EN 15986, Symbol for use in the labeling of medical devices Requirements for labeling of medical devices containing phthalates as described in Section 4.2 of the Standard |
Contains or presence of phthalate DEHP | This product contains Di(2-ethlhexyl)phthalate (DEHP) which has been shown to cause reproductive harm in male neonates, pregnant women carrying male fetuses and peripubertal males. The following procedures have been identified as posing the greatest risk for DEHP exposure: exchange transfusion in neonates, total parenteral nutrition (TPN) in neonates (with lipids in polyvinylchloride (PVC) bag), multiple procedures in sick neonates (high cumulative exposure), heart transplantation or coronary artery bypass graft surgery (aggregate dose) and massive infusion of blood into trauma patient. It is recommended that DEHP-free medical products be considered when these procedures are to be performed on male neonates, pregnant women who are carrying male fetuses and peripubertal males. | |
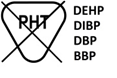 |
BS EN 15986, Symbol for use in the labeling of medical devices Requirements for labeling of medical devices containing phthalates as described in Section 4.2 and Annex B of the Standard |
Does not contain the phthalate plasticizers DEHP, DIBP, DBP or BBP | Indicates product that does not contain the phthalate plasticizers DEHP, DIBP, DBP or BBP. | |
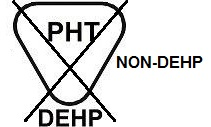 |
BS EN 15986, Symbol for use in the labeling of medical devices Requirements for labeling of medical devices containing phthalates as described in Section 4.2 and Annex B of the Standard |
Does not contain the phthalate plasticizer DEHP | Indicates product that does not contain the phthalate plasticizer DEHP. |